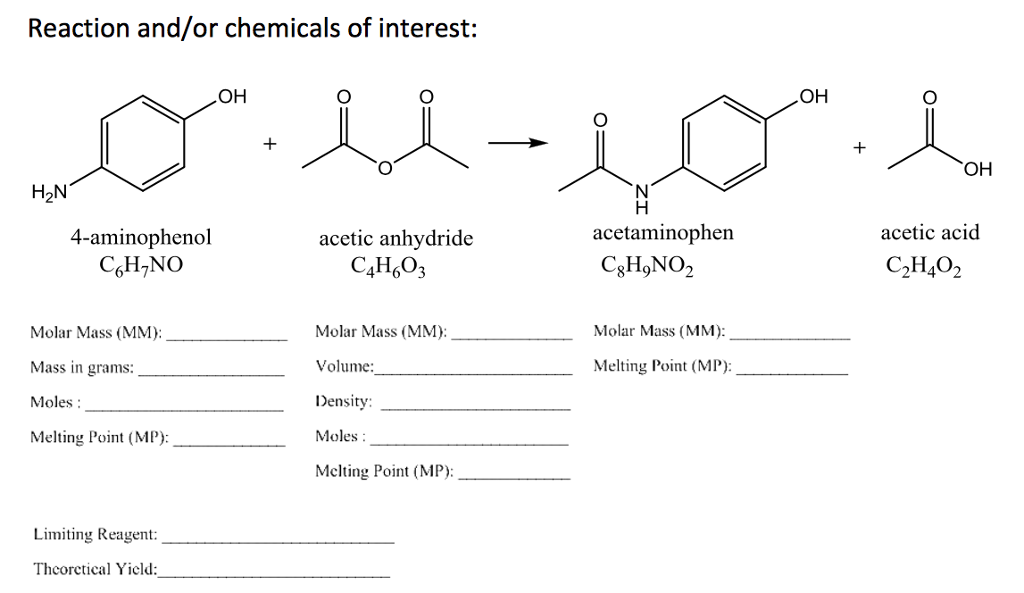
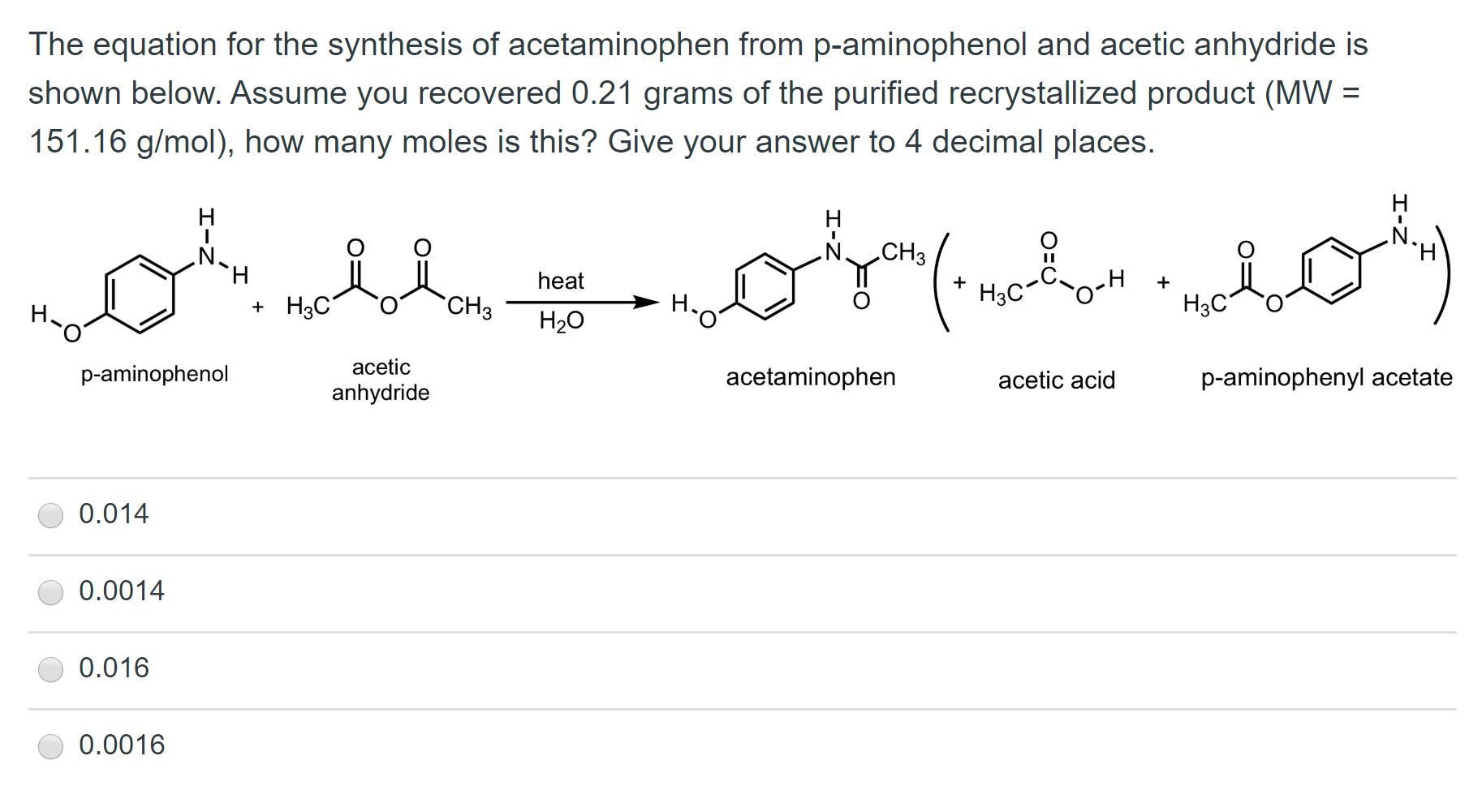
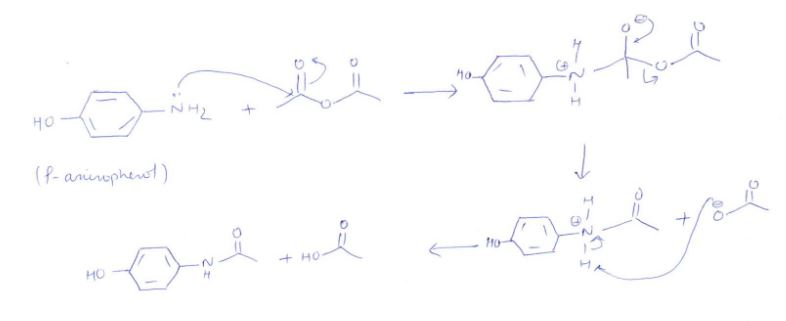
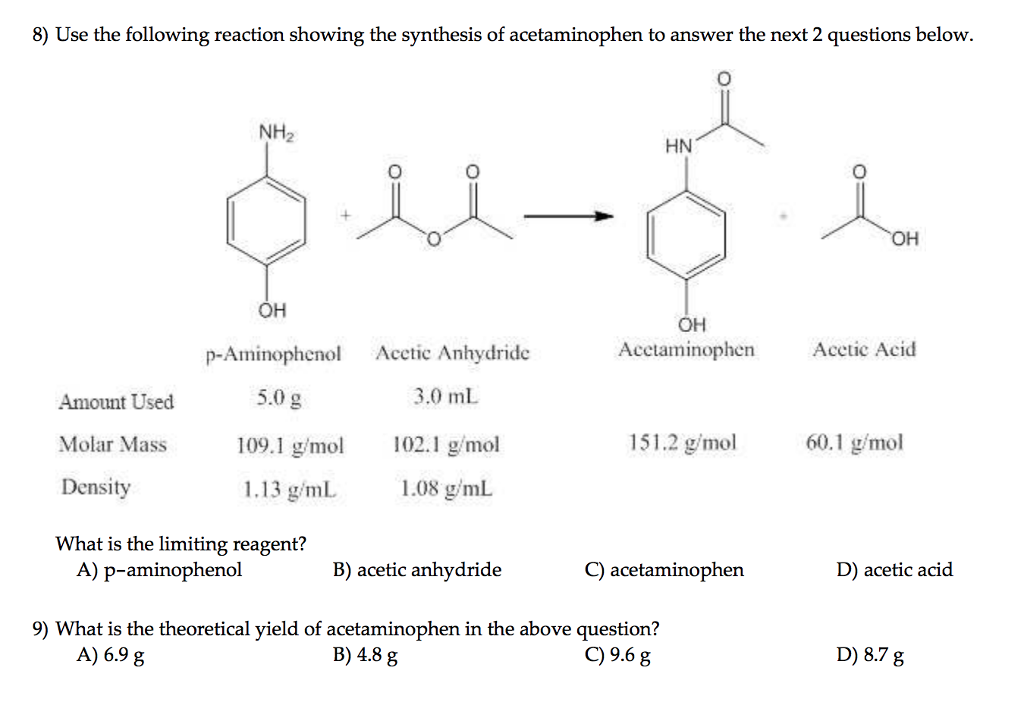
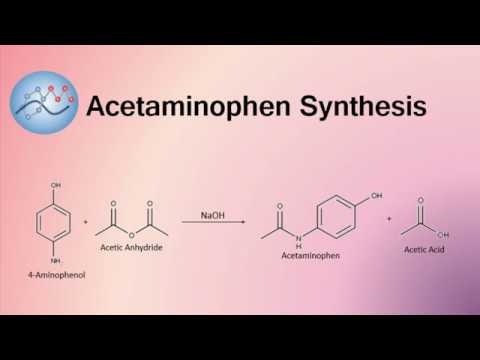
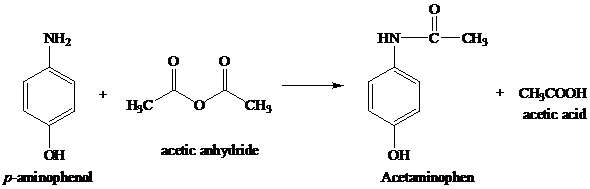organic chemistry - While treating 4-aminophenol with acetic anhydride why does the reaction stop at Paracetamol and does not undergo esterification with acetic acid? - Chemistry Stack Exchange

How can I draw a curly arrow mechanism for the functional group interconversion reaction between p-aminophenol and acetic anhydride? | Homework.Study.com
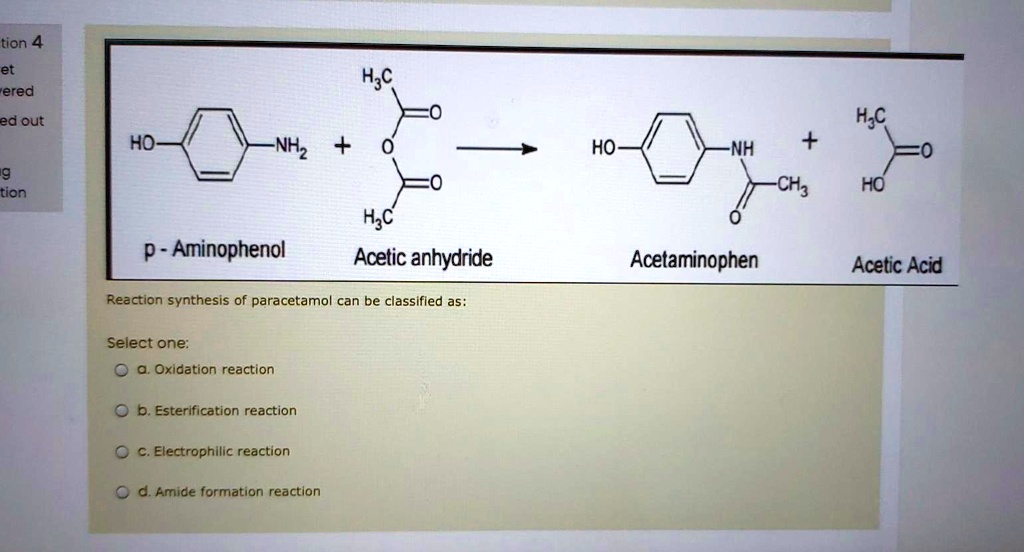
SOLVED: tion 4 H;c ered ed out Hjc HO NHz HO NH + CHz tion HO H;c Acetic anhydride P - Aminophenol Acetaminophen Acetic Acid Reaction synthesis of paracetamol can be classified
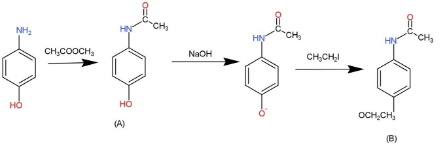
When p- aminophenol reacts with one molar equivalent of acetic anhydride, a compound A $({{C}_{8}}{{H}_{9}}N{{O}_{2}})$is formed that dissolves in dilute NaOH. When A is treated with one equivalent of NaOH followed by
✓ Solved: In the reaction between p-aminophenol and acetic anhydride to form acetaminophen, 4.5mL of...
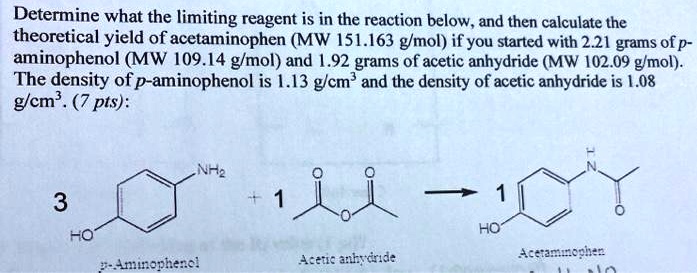
SOLVED: Determine what the limiting reagent is in the reaction below, and then calculate the theoretical yield of acetaminophen (MW 151.163 gmol) if you started with 2.21 grams ofp aminophenol (MW [09.14

1. a. Write the balanced chemical equation for the synthesis of acetaminophen from p-aminophenol and acetic anahydride. b. Starting with 1.5 grams of p-aminophenol and an excess of acetic anhydride, ...

Which of the following statements are true regarding the reaction between p- aminophenol and acetic anhydride. 1. As the reaction proceeds, p-aminophenol loses a hydrogen ion from its amine group. 2. As the